Alcohols, anesthesia and neutron scattering
By Mária Klacsová, Daniela Uhríková and Pavol Balgavý
1-alcohols (CnOH) with a number of carbon atoms (n) lower than 14 are general anesthetics [1]. Lipid theories of anesthesia suggest that the bilayer of biomembranes is the primary target of anesthetics. Bilayer structural perturbations induced by anesthetics affect membrane protein conformations and result in functional changes in the proteins.
In the bilayer, the OH group of CnOH is located at the level of phospholipid polar groups and the CnOH alkyl chain extends into the bilayer´s hydrophobic interior. At a constant alcohol concentration in the bilayer, the mismatch between shorter alcohol and longer lipid hydrocarbon chains should result in a decrease in the bilayer thickness; this decrease should diminish with the alcohol chain length increase up to the lipid length.
The aim of our investigations was therefore to study bilayer thickness as a function of CnOH chain length n. The bilayer thickness can be obtained from small-angle neutron scattering (SANS) on unilamellar phospholipid vesicles (ULV) [2, 3]. We performed this experiment on PAXE spectrometer in LLB Saclay.
SANS experiment on PAXE
We prepared ULVs from dioleoylphosphatidylcholine (DOPC) with small amount (4 wt. %) of dioleoylphosphatidylserine (DOPS). We dispersed solid DOPC+DOPS+CnOH mixtures in D2O + H2O mixtures (outer contrasts) and extruded these dispersions through 50 nm pores in 2 stacked carbohydrate filters. We measured SANS spectra on PAXE (sample to detector distance 1.3 m and 5.05 m, λ=0.6 nm) at 25ºC.
The extruded ULVs are polydisperse hollow spheres with a single bilayer separating the inside and outside aqueous compartments. We divided the bilayer into three strips corresponding to two polar headgroup regions and the bilayer center spanning hydrocarbon region. The scattering length densities of polar and hydrophobic regions were calculated using the known scattering lengths and component volumes of DOPC, DOPS and CnOH were measured by densitometry.
Effect of CnOH on bilayer thickness and area
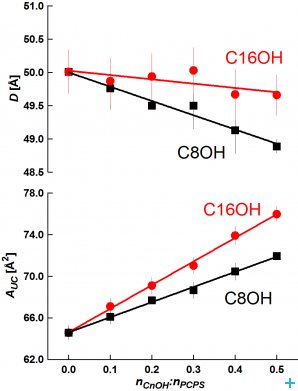
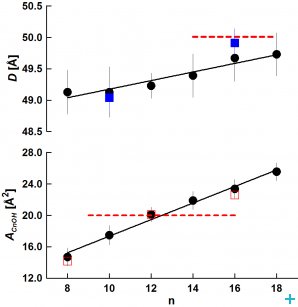
From SANS data, we evaluated the bilayer thickness, D, as well as the lateral area of the unit cell, which consists of a phospholipid molecule and a particular fraction of the alcohol at the bilayer–aqueous phase interface, AUC (see [2, 3]). For illustration, selected D results as a function of CnOH:lipid molar ratio (Figure 1) and as a function of CnOH chain length n at fixed CnOH:lipid=0.4 molar ratio (Figure 2) are shown and compared with control value of D (dashed line) obtained at CnOH:lipid=0:1 molar ratio.
As we predicted, the thickness D decreases due to CnOH and lipid chain length mistmatch; this effect is larger at higher CnOH concentrations and diminishes with the CnOH chain length n. The interface area AUC increases due to CnOH intercalation between lipid molecules. From the data, we have also calculated the interface area of CnOH, ACnOH, in bilayers (Fig. 2). Anomalously small values of ACnOH were obtained for n<12 – smaller than the chain cross-section area ~20 Å2 (dashed line) in solid rotator phases of n alkanes. We suppose that this anomaly is caused by the lipid headgroup whose interface area is larger or equal comparing to the sum of hydrocarbon chains cross section areas, so that a small OH group is located underneath at the lipid glycerol fragment. Simulations of DOPC+CnOH bilayers carried out with the GROMACS molecular dynamics package, using the MARTINI coarse-grained force field, reproduced our experimental results [3]. Dr. M. Bulacu and Professor S.J. Marrink from the University of Groningen performed these simulations.
References:
1. Cantor R.S.: Biophys. J. 80, 2001, 2284
2. Kučerka N., Nagle J.F., Feller S.E., Balgavý P.: Phys. Rev. E 69, 2004, 051903
3. Klacsová M., Bulacu M., Kučerka N., Uhríková D., Teixeira J., Marrink S.J., Balgavý P.: Biochim. Biophys. Acta 1808, 2011, 2136
Dr. M. Klacsová was a PhD student of biophysics (2006-2010) and Prof. P. Balgavý her supervisor at the Department of Nuclear Physics and Biophysics, Faculty of Mathematics, Physics and Informatics, Comenius University in Bratislava, Assoc. Prof. D. Uhríková is the Head of Department of Physical Chemistry of Drugs, Faculty of Pharmacy, Comenius University in Bratislava and Prof. P. Balgavý and Dr. M. Klacsová are staff members of this Department.